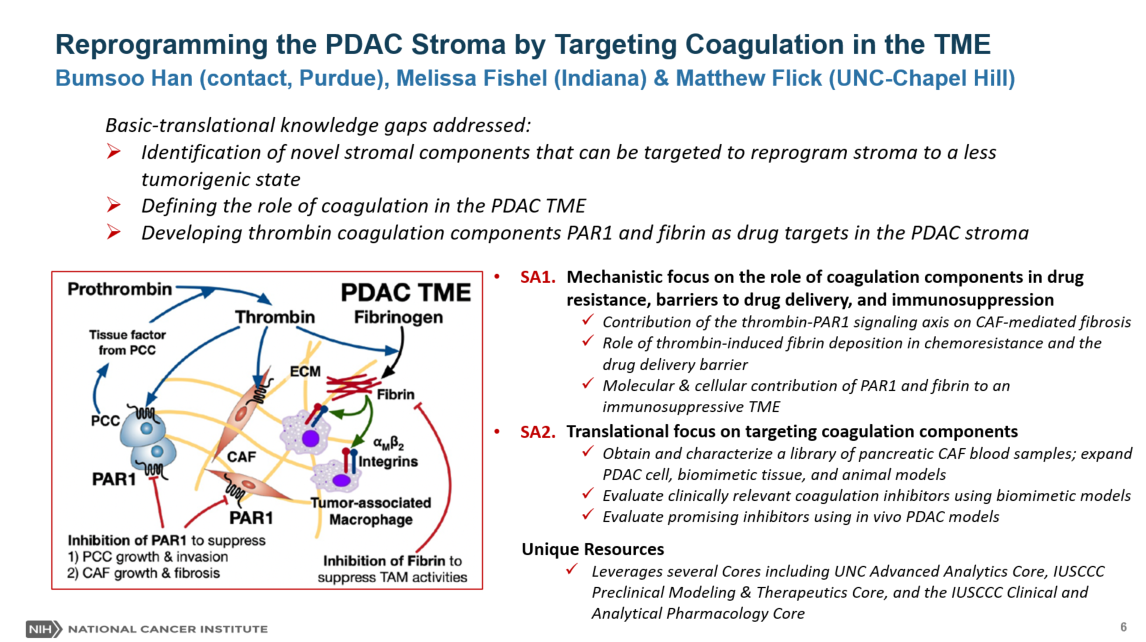
The overarching goal of this research program is to identify therapeutic strategies to convert the stroma of pancreatic ductal adenocarcinoma (PDAC) to a chemo- and immune-sensitive tumor microenvironment (TME). PDAC is characterized by a desmoplastic stroma that facilitates tumor growth/invasion, chemoresistance of pancreatic cancer cells (PCC), and immunosuppressive TME. Highly packed cancer-associated fibroblasts (CAFs) and dense extracellular matrix (ECM) are hallmarks of the PDAC stroma and constitute physical drug delivery barriers. Several stromal components have been targeted to enhance drug delivery, but recent studies have suggested anti-tumor roles for the stroma as complete ablation of stromal components leads to more aggressive tumors. New strategies are highly desired to reprogram stroma without compromising its anti-tumor roles. The central hypothesis is that the coagulation system in the PDAC TME can be targeted to reprogram PDAC stroma to overcome chemoresistance, drug delivery barriers, and immunosuppressive TME. Cancer-associated coagulation has been reported as a key functional signaling pathway in PDAC. Notably, several coagulation molecular targets, including thrombin, protease-activated receptor 1 (PAR1), and fibrinogen/fibrin, have been implicated in important roles contributing to tumor progression and therapeutic resistance. Specifically, it is hypothesized that the thrombin-PAR1 signaling axis can be targeted to suppress PCC growth/invasion and CAF growth/fibrosis. In addition, thrombin-mediated fibrin deposition can be targeted to suppress the drug delivery barrier and immunosuppressive tumor-associated macrophage activities. This hypothesis will be tested and evaluated for translational potential by pursuing the following two integrated aims: Aim 1) Mechanistic Research: Determine the contribution of the coagulation targets in the PDAC TME. Specifically, the team will determine the role of thrombin-PAR1 signaling axis to CAF-mediated fibrosis, thrombin-mediated fibrin deposition on drug resistance, and PAR1/fibrin on the immunosuppressive TME. Aim 2) Translational Research: Evaluate the pharmacological inhibition of the coagulation targets. Especially, the team will expand the mechanistic understanding from Aim 1 using patient-derived PDAC models with FDA-approved inhibitors of thrombin and PAR1, and fibrinogen depleting agents. The effects of pharmacological inhibition will feedback to Aim 1 to delineate the efficacy of inhibiting coagulation targets. The outcome of this research will establish a new mechanistic understanding of the role of coagulation activities in the PDAC TME. It will determine whether blockade of the coagulation is a promising strategy to reprogram the PDAC stroma and, ultimately, suppress PCC/CAF growth and improve drug delivery and efficacy. For more information click here

Bumsoo Han is a Professor of Mechanical Engineering and Biomedical Engineering at Purdue University. He is also a Program Co-Leader of Drug Delivery and Molecular Sensing Program of NCI-designated Purdue Institute for Cancer Research. He received his Ph.D. in Mechanical Engineering from the University of Minnesota, and his M.S. and B.S. from Seoul National University in Korea. After his Ph.D., he was a Postdoctoral Research Associate in Mechanical and Biomedical Engineering at the University of Minnesota. His broad research interests are in biotransport for drug delivery and tissue engineering. His current research efforts are focused on the transport of drugs and nanoparticles at the tumor microenvironment, specifically pancreatic cancers, disease-on-chip models, and cell-fluid-matrix interaction in stromal tissues. He received US DOD Postdoctoral Award for Breast Cancer Research, NSF CAREER Award, Faculty Fellowship from U.S. Air Force Research Laboratory (Predictive Toxicology Program), and Richard Skalak Best Paper Award from ASME Journal of Biomechanical Engineering. He is a recipient of the Faculty of Excellence Early Career Research Award from Purdue University. He is a Fellow of ASME. For more information click here.


Our role in the Blood Research Center (BRC) is to provide a unique perspective on the contribution of coagulation and fibrinolytic factors to a wide spectrum of disease pathologies, independent of the traditional roles of these factors in bleeding and thrombosis. We utilize mouse models to analyze specific mechanisms by which proteins such as prothrombin, fibrinogen, and plasminogen contribute to the progression of inflammatory, infectious, and malignant disease. Working with our collaborative basic science and clinical partners in the BRC provides an unparalleled opportunity for rapidly advancing our understanding of the multifaceted role of the hemostatic system in hemostasis, thrombosis, and beyond. For more information click here.

Dr. Timothy Ratliff, PhD, is a distinguished professor in comparative pathobiology and the Robert Wallace Miller Director of the Purdue University Center for Cancer Research. He received his BS in 1971 from the University of Texas; his MS from Texas A&M; and his PhD from the University of Arkansas I 1977. The Purdue University Center for Cancer Research has a fundamental approach to discover new information about cancer that will lead to new ways to detect and treat the disease.

Dr. Chi Zhang focuses on computational modeling of cancer micro-environment including the level of hypoxia, oxidative stress, acidity and dysregulation of extracellular matrix as well as altered immune responses in cancer tissue by using large scale omics data. Dr. Chi Zhang is also interested in developing novel computation methods to integrate multiple tissue level and single cell omics data types to understand the mechanism of cancer initiation, progression, metastasis and cancer tumor tissue’s resistance to certain therapies. In addition, his research application also includes inference of intra-tumor heterogeneity and reprogrammed metabolism, and prediction of gain or loss of functions led by a certain mutation or collective effect of multiple mutations. Chi Zhang's lab webpage: https://bdr.netlify.com

Anita Turk is a Hematologist Oncology expert in Indianapolis, Indiana. Turk has been practicing medicine for over 11 years and is highly rated in 5 conditions, according to our data. Her top areas of expertise are Pancreatic Cancer, Familial Pancreatic Cancer, Gallbladder Cancer, Gallbladder Adenocarcinoma, and Bone Marrow Aspiration. Turk is currently accepting new patients.
Her clinical research consists of co-authoring 9 peer reviewed articles and participating in 11 clinical trials in the past 15 years.