Pancreatic cancer is a deadly malignancy, direly in need of new therapeutic approaches. Pancreatic cancer is characterized by extensive accumulation of a fibroinflammatory stroma, containing abundant fibroblasts. Long believed to be a uniform cell population, fibroblasts have emerged as a heterogeneous and functionally important component of the tumor. Yet, the literature on fibroblasts in pancreatic cancer is controversial, with studies supporting both a pro- and an anti-tumor role. Our preliminary data show that oncogenic KRAS, a hallmark mutation of pancreatic cancer, expressed in tumor cells extrinsically reprograms the transcriptional program of fibroblasts. Further, continuous expression of epithelial oncogenic Kras is required to maintain expression of a panel of inflammatory cytokines in fibroblasts. Unlike mouse tumors, human pancreatic cancer is highly heterogeneous in terms of genetic alterations and histological characteristics of cancer cells. How fibroblasts are reprogrammed in the context of different types of human pancreatic cancer and how they contribute to carcinogenesis is unclear. Lastly, most in depth studies on pancreatic cancer are based on largely White patient populations, while the disease is equally prevalent in all race groups. The University of Michigan/Henry Ford Health System team together has access to a large patient volume, diverse patient populations (with 30% HFHS patients being African American), and the skillset to characterize and functionally assess fibroblasts on pancreatic cancer. We will map fibroblast heterogeneity across tumors in a diverse patient population. We will evaluate whether fibroblast heterogeneity correlates with different subtypes of tumor cells (classified as classical and mesenchymal). We will further take advantage of the genetically engineered mouse models available in our group to target fibroblast reprogramming at different stages of carcinogenesis. Overall, our work will shed light on the regulation and function of pancreatic CAFs and open the way for new therapeutic approaches targeting this population. For more information click here
https://pancreas.med.umich.edu/ https://twitter.com/UMICHpancreas https://pancreas.med.umich.edu/lyssiotis-lab/
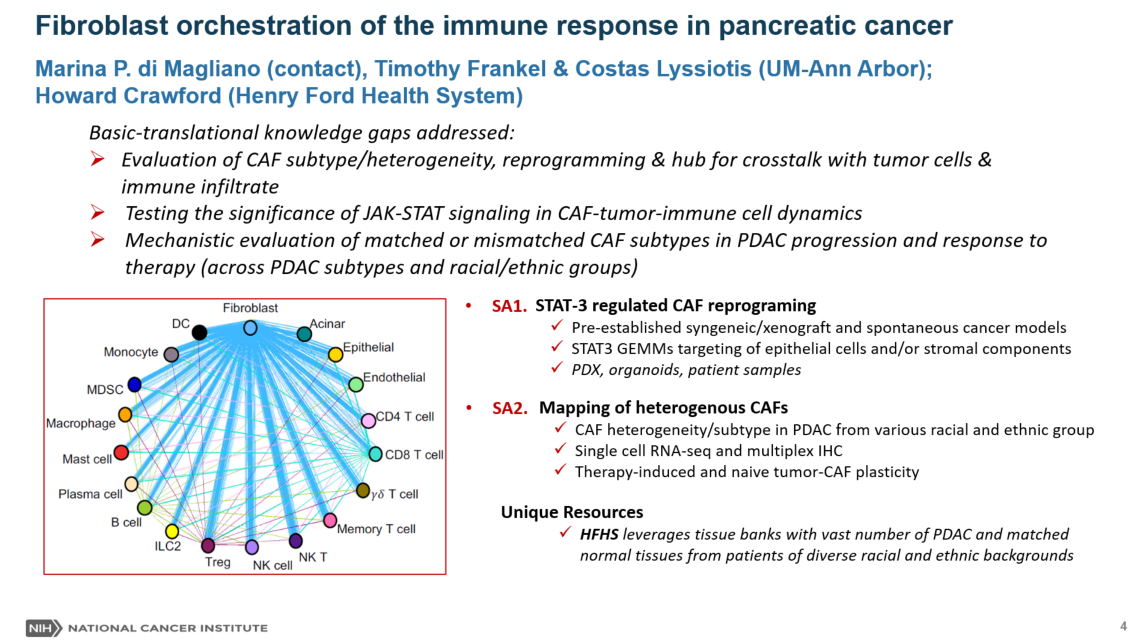

Dr Marina Pasca di Magliano is the Maude T Lane Professor or Surgery, Professor of Cell and Developmental Biology at the University of Michigan. Dr Pasca di Magliano is the co-leader of the Signaling and Tumor Microenvironments Program for the Rogel Cancer Center and co-director of the Pancreatic Disease Initiative. The Pasca di Magliano Laboratory investigates the cellular crosstalk in the pancreatic cancer microenvironment. We have a longstanding interest in understanding how oncogenic KRAS regulates features of the precursor microenvironment (PME) and tumor microenvironment (TME). We use genetically engineered mouse models, patient samples and organoids and a combination of wet lab and computational approaches to dissect the interactions between cancer cells, fibroblasts and immune cells, and study how these interactions are affected by treatment. The long-term goal of our research program is to identify new ways to target the microenvironment in pancreatic cancer patients.

Dr. Howard Crawford is the Scientific Director of the Henry Ford Pancreatic Cancer Center (HFPCC) in Detroit, and holds faculty positions at the University of Michigan, Michigan State University and Wayne State University. The Crawford lab focuses on epithelial plasticity and the associated triggering of the host reaction in pancreatitis, pancreatic tumorigenesis, and tumor progression. Dr. Crawford oversees the use of pancreas samples collected from a ethnically diverse patient population in Detroit.

Dr. Timothy L. Frankel is a faculty member within the Section of General Surgery at the University of Michigan. He graduated Magna Cum Laude from Connecticut College in 2000 with a B.A. in Physics and was elected into Phi Beta Kappa. He earned his medical degree from George Washington University in 2004 and was inducted into the Alpha Omega Alpha Honor Medical Society. He completed a residency in General Surgery at the University of Michigan in 2011 during which time he spent two years at the National Cancer Institute studying tumor immunology and genetic engineering. He developed techniques to genetically modify peripheral blood lymphocytes allowing them to more effectively target tumors for destruction. His work resulted in multiple publications and awards including the James Crudup Surgical Research Award. After completion of residency, he completed a two-year surgical oncology fellowship at Memorial Sloan-Kettering Cancer Center in New York.

Dr. Costas Lyssiotis is the Maisel Research Professor of Oncology, associate professor of molecular and integrative physiology, and of internal medicine, Medical School; and co-director of the Pancreatic Disease Initiative and the Rogel Cancer Center Tumor & Immune Metabolism Program. The Lyssiotis lab investigates biochemical pathways and metabolic requirements that enable tumor survival and growth. This work spans the areas of cancer metabolism, the tumor microenvironment and immunometabolism. Ultimately, his group aims to transition new information about these processes into targeted therapies for cancer and other diseases. He is also passionate about science education and training the future generation of scientists. He serves as the director of U-M’s Graduate Program in Cancer Biology, and he is an active lecturer and participant in graduate education.
Additional URL:
 Lyssiotis lab
Lyssiotis lab